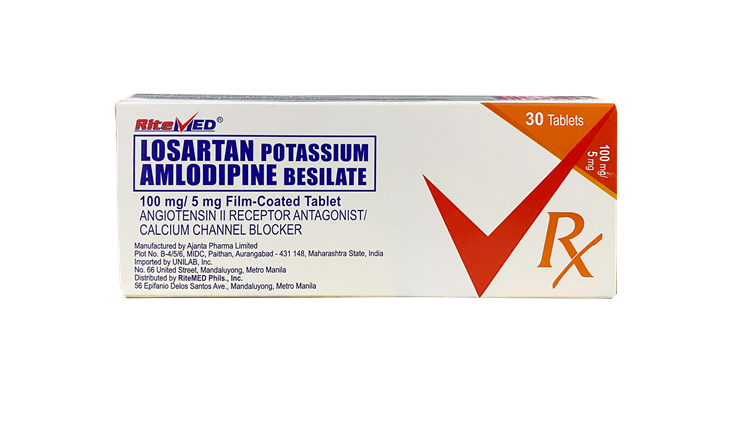
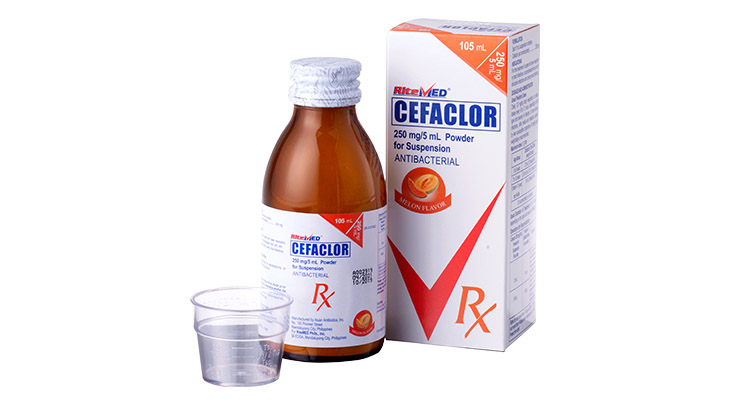
Product Details
What is this medicine used for?
For the treatment of the following infections by susceptible microorganism: Skin and skin structure infections. Bone and joint infections. Genitourinary tract infections, including acute prostatic. Respiratory tract infection including acute and chronic bronchitis and infected bronchiectasis. Ear, nose and throat infection including otitis media, mastoiditis, sinusitis, follicular sinusitis, and pharyngitis. Dental infections.
How much and how often should you use this medicine?
Use in adult: 250mg every 6 hours ( Maximum dose: 4g/day) Usual Pediatric Dose: 25 to 50 mg/kg body weight/day to be given in equally divided doses every 6 hours.
Contraindication:
Known hypersensitivity to cephalosporins, penicllins, or any component of the product.
Warnings and Precautions:
Careful inquiry should be made prior to cefalexin therapy to determine whether before the patient has had previous hypersensitivity reactions to cefalexin, cephalosporins, penicillins, or other drugs. Use with caution in patient with penicillin-sensitive patients since cross-sensitive among beta lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of allergy to penicillin. In case of an allergic reaction to cefalexin, the drug should be discontinued. Serious acute hypersensitivity reactions may require immediate treatment with epinephrine and other emergency measures, including oxygen, intravenous fluids, corticosteroids, pressor amines, and airway management, as clinically indicated. Clostridium difficile-associated diarrhea (CDAD) and colitis have been reported with the use of nearly all antibacterial agents, including amoxicillin, and may range in severity from mild to life threatening. It is important to consider this diagnosis in patients who present with diarrhea following administration of antibacterial agents. Cephalosporins, including cefalexin, have been associated with the development of seizures, particularly in patients with renal impairment, in whom dosage of drug was not reduced. If seizures due to cefalexin develop, the drug should be discontinued and the treatment with an anticonvulsant be given as clinically indicated. Cephalosporins may be associated with a fall in prothrombin activity. Patients who are at risk are those with renal or hepatic impairment or poor nutritional state, as well as patients receiving protracted course of antimicrobial therapy, and patients stabilized on anticoagulant therapy. prothrombin time should be monitored in patients at risk and exogenous Vitamin K administered as indicated. A false-positive comb's test has been reported during treatment with other cephalosporins antibiotics; therefore, it should be recognized that a positive coombs test may be due to the drug, e.g., coombs testing of new burns whose mother has receive cephalosporins before parturitions or in hematologic studies or in transfusion cross-matching procedures where anti- globulin test are performed. Use with caution in patients with renal impairment. Careful clinical and laboratories would be made since safe dosage may be lower than that usually recommended. Indicated surgical procedures. As any potent drug, periodic assessment of organ system function, including renal, hepatic , hematopoietic, is recommended during prolonged therapy. Prescribing cefalexin in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to patient and increase the risk of antibiotic resistance. As with other antibacterial drugs, long term or repeated use may result in overgrowth in non-susceptible organisms, including fungi.
Undesirable Effects:
GI: abdominal cramp/pain, anorexia, CDAD, and colitis, diarrhea, dyspepsia, gastritis, gastroenteritis, glossitis/stomatitis, nausea, vomiting. Dermatologic: Anaphylaxis, angioedema, eosinophilia, erythema multiforme, fever, genital and anal pruritis, intertrigo, pruritus, rash, serum sickness-like reactions, Stevens-Johnson syndrome, toxic epidermal necrolysis, urticaria. Hematologic: Decreased hemoglobin and/or hemotocrit, hemolytic anemia, reversible leukopenia; neutropenia, decreased platelets; positive direct and in direct ant globulin test results; thrombocytopenia. Renal and Genitourinary: Transient increase in blood urea nitrogen(BUN) and serum creatinine concentrations; dysuria, reversible interstitial nephritis, nephrotoxicity, leukorrhea, pruritus vulvae, vaginal discharges, vulvo-vaginitis. Hepatic; Transient increase in serum alanine aminotransferase(ALT), aspartate aminotransferase(AST), and alkaline phosphatase concentrations; increased serum bilirubin; transient hepatitis and cholestatic jaundice. Nervous System; agitation, confusion, diplopia, dizziness, fatigue, headache, hallucinations, insomnia, malaise, paresthesia, somnolence, tremor, toxic paranoid reactions. Vertigo, tinnitus, hearing loss and behavioral changes in young children have been reported with cefalexin use. Other AE; Arthralgia, myalgia, arthritis, joint disorder back pain, nuchal swelling, cardiac arrhythmia, vasodilation, dyspnea, superinfection, elevated cholesterol.
Interaction w/ other medicaments:
No potentially hazardous interactions has been reported. However, it may share the interaction of other cephalosporins as with the following: Alcohol: Disulfiram-like reactions have occurred when alcohol was ingested with 48-72 hours after administration of beta lactam antibiotics that contain n-methylthiotetrazole side chain. The reactions appear to result from accumulation of acetaldehyde and do not occur if alcohol is ingested prior to the first dose of antibiotic ingestion of alcohol during 24 to 72 hours after administration of some cephalosporins should be avoided. Estrogen or Progestin: Some cephalosporins reabsorption and reduced efficacy of oral contraceptives. Metformin: when single 500 mg doses of cefalexin and metformin were given to 12 healthy subjects, plasma metformin Cmax and AUC increased by an average 34% and 24%, respectively, the metformins are multiple renal clearance decreased by an average of 14%. Information on the interaction of cefalexin and metformin after multiple doses of administration is unavailable. Careful patient monitoring and metformin dose adjustment is recommended during concomitant therapy. Nephrotoxic drugs: Concurrent use of nephrotoxic agents such as aminoglycosides, colistin, polymixin B, or vancomycin may increase the risk of nephrotoxicity with some cephalosporins and probably be avoided, if possible. Diuretics: concurrent treatment with high doses of cephalosporins and potent diuretics (e.g., furosemide) may adversely affect renal function.